









A very important quantum mechanical principle is the uncertainty principle. It says that there are certain quantities that we cannot measure precisely at the same time. The first inequality is the most famous version of the uncertainty principle. It states that there is a limit to how accurately we can measure the position and the momentum in a system simultaneously. The second inequality is another version of the uncertainty principle that is less well known. It states that we can only measure the energy in a system to a certain degree of accuracy over a given time interval. This version will be important for some of the things that we discuss later in the talk.
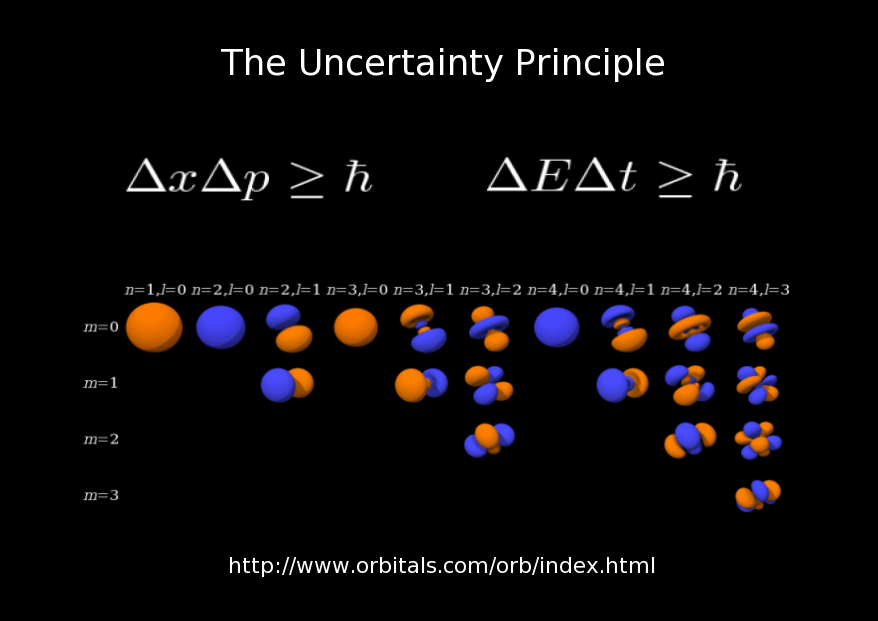
As a practical example of a consequence of the uncertainty principle, I have included these images of atomic orbitals. Due to the uncertainty principle, the electrons belonging to an atom can literally be anywhere. These surfaces are the best that we can do in terms of specifying where the electrons reside around the nuclei. They are the surfaces where it is most likely that the electron will be. The table is organized according the quantum numbers n, l, and m that specify the orbitals. The first quantum number, n, is related to the energy of the electrons and the other two quantum numbers, l and m, specify the angular momentum of the electrons.
I am the author of the images and text except where otherwise indicated. Please contact me for permission if you wish to use any of my images or text.
Created on Wednesday 03 May 2006 by Mark A. Martin with KPresenter